
Has been best completed for the hydrogen atom. To the idea of an orbital and the best way to think about orbitals is to think about a hydrogen atom and actually the map for orbitals, it's hydrogen as the simplest atom and so the map for orbitals And so to describe whereĮlectrons are likely to be found, physicist and chemists introduced That it's there's, some probability that it's over there. Some probability it's here, there's some probability that it's there, there's some probability In fact, at any given point in time, it's not necessarily exactly right there, it could be there but there's Well-defined circular or elliptical orbits. I'm trying to just draw an elliptic or a circular looking orbit. So maybe one electron has an orbit that looks something like that,Īnd then another electron, if we were talking aboutĪ neutral helium atom will have two electrons and two protons, well maybe, the other one The way that a planet would orbit around its star. Which has a negative charge orbits around the nucleus Protons have a positive charge, "electrons have a negative charge, "so they'll be attracted to each other." Opposite signs, opposite charges attract. And early physicists and chemists said, "All right, well, if the Would have two neutrons as well so the nucleus might Protons in the nucleus and a typical helium atom

Which is made up of the protons and the neutrons isĬoncentrated at the center and so the early modelįor how an atom worked was maybe you have your protonsĪnd neutrons in the center so let's say, we're talkingĪbout a helium atom. Physicist and chemists were facing over a hundred years ago is how are these things configure and they realized that the positive charge is concentrated at the center of the atom. Which have neutral charge or no charge and then you have your electrons And those particles are the protons which have positive charge, you have your neutrons For example, the reason all three 2p orbitals must have the same energy is because they’re all related to one another by rotations.Learned in other videos that the atom is in fact made up of even smaller constituent particles which is pretty amazing because atoms are already It is actually the spherical symmetry that is responsible for some of the degeneracy of the orbitals.
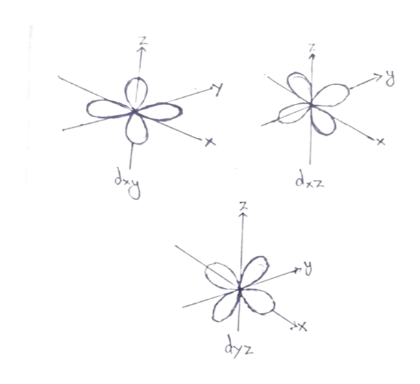
This is why it is the orbitals with non-zero values of the angular momentum quantum number (p,d,f etc.) that break the symmetry, whereas the l=0 s orbitals are spherically symmetric.įinally, it’s worth mentioning that the spherical symmetry is present in the solution, but just in a slightly different form. Quantum mechanically, the same kind of principles apply, but of course due to uncertainty, it’s no longer possible to construct an angular momentum vector in quite the same way. Classically, angular momentum is a conserved vector, which means that any orbit with non-zero angular momentum has a special axis, the axis aligned with its angular momentum. The actual culprit of the symmetry breaking is the same in each case, orbital angular momentum. The Earth’s orbit isn’t spherically symmetric, instead it’s confined to a single plane (or equivalently the about is always about a specific axis), so it would actually be very weird if the quantum mechanical version of the same problem was entirely spherically symmetric. Perhaps it might be helpful for you to remember that an electron orbiting a proton is essentially a quantum mechanical version of the Earth orbiting the Sun.


 0 kommentar(er)
0 kommentar(er)
